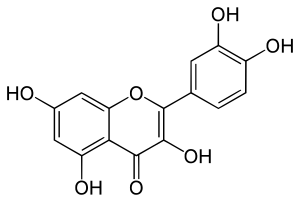
Quercetin
Quercetin is a naturally occurring flavonoid found in a wide variety of fruits, vegetables, and grains. It is one of the most abundant antioxidants in the human diet and plays a significant role in helping to protect cells from damage caused by free radicals.
Quercetin belongs to the class of polyphenolic compounds known as flavonoids, recognized for their diverse biological activities. It is a pigment that is responsible, in part, for the colors of many fruits, vegetables, and flowers. Structurally, quercetin is characterized by the presence of a 15-carbon skeleton consisting of two phenyl rings and a heterocyclic ring.
The term "quercetin" is derived from quercetum (oak forest), reflecting its presence in oak trees. It was first isolated in 1857 by the Austrian chemist Heinrich Hlasiwetz. Since its discovery, quercetin has been the subject of extensive research, with numerous studies exploring its potential health benefits, primarily due to its antioxidant, anti-inflammatory, antiviral, and anticancer properties.
In plants, quercetin serves as a protective substance, defending against microbial infections and environmental stress. This feature positions quercetin as a xenohormetic compound, implying that it not only protects the plants producing it but also potentially offers health benefits to humans who consume these plants. For humans, it’s predominantly obtained through the consumption of foods such as onions, apples, berries, and teas. Its biological roles in human health are vast, with studies suggesting benefits ranging from anti-aging and anti-inflammatory effects to potential protective roles against various diseases.
Natural Occurrence and Derivatives
Quercetin is naturally found in various plants, primarily as glycosides, where the quercetin molecule is bound to a sugar molecule, enhancing its solubility and transport within the plant. These glycosides are metabolized to free quercetin in the human digestive system when consumed through the diet. Notable sources of quercetin include onions, apples, berries, and tea, among others. The presence of quercetin in these forms in plants underscores its relevance in plant physiology, particularly in providing protection against oxidative stress and contributing to the pigmentation in plant tissues.
| Foods | Quercetin Content (mg / 100 g) |
|---|---|
| Capers (Raw) | 234[1] |
| Capers (Canned) | 173[1] |
| Lovage Leaves (Raw) | 170[1] |
| Dock (Sorrel-like) | 86[1] |
| Radish Leaves | 70[1] |
| Carob Fiber | 58[1] |
| Dill Weed (Fresh) | 55[1] |
| Coriander | 53[1] |
| Yellow Wax Pepper (Raw) | 51[1] |
| Fennel Leaves | 49[1] |
| Onion (Red) | 32[1] |
| Radicchio | 32[1] |
| Watercress | 30[1] |
| Kale | 23[1] |
| Chokeberry | 19[1] |
| Bog Blueberry | 18[1] |
| Buckwheat Seeds | 15[1] |
| Cranberry | 15[1] |
| Lingonberry | 13[1] |
| Plums (Black) | 12[1] |
In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration.[2] One study found that organically grown tomatoes had 79% more quercetin than non-organically grown fruit.[3] Quercetin is present in various kinds of honey from different plant sources. [4]
Background Intake
Quercetin is one of the most abundant dietary flavonoids. [5][1] To estimate the average quercetin intake of the U.S. population, data from the flavonoid database was combined with food consumption data from the 2003-2004 National Health and Nutrition Examination Surveys (NHANES). Only individuals who reported consuming foods naturally rich in quercetin were included in the analysis.
The table below presents the estimated daily quercetin intake from natural sources for various U.S. population groups. The overall consumption of quercetin-rich foods led to an estimated average intake of 5.9 mg/person/day, with the 90th percentile at 14.7 mg/person/day. The highest reported individual intake was 258.2 mg/day, observed in the adult male group. It's worth noting that these estimates might be on the conservative side, as quercetin content data is available only for select foods.
| Population Group | Age Group (Years) | % Users | Background Quercetin Intake (Users Only) | ||
|---|---|---|---|---|---|
| Mean (mg) | 90th Percentile (mg) | Maximum (mg) | |||
| Infants | 0 - 2 | 54.2 | 1.9 | 3.7 | 60.2 |
| Children | 3 - 11 | 85.5 | 2.6 | 6.3 | 96.4 |
| Female Teenagers | 12 - 19 | 79.6 | 4.9 | 10.7 | 176.8 |
| Male Teenagers | 12 - 19 | 83.9 | 6.1 | 15.1 | 118.2 |
| Female Adults | 20+ | 82.4 | 5.7 | 15.6 | 117.5 |
| Male Adults | 20+ | 84.0 | 7.6 | 17.8 | 258.2 |
| Total Population | All Ages | 79.9 | 5.9 | 14.7 | 258.2 |
Chemical and Physical Properties
Quercetin possesses distinct chemical and physical properties, integral to its bioactivity and its interaction within biological systems.
- Molecular Structure: Quercetin has a molecular formula of C15H10O7 and is composed of two phenyl rings (A and B) bonded to a heterocyclic ring (C) containing one oxygen atom, forming a structure typical of flavonoids. Its full chemical name is 3,3',4',5,7-pentahydroxyflavone.
- Solubility and Stability: Quercetin has limited solubility in water but is soluble in organic solvents like ethanol. It is relatively stable in acidic conditions but is susceptible to degradation in basic conditions and under exposure to light and heat. Its stability is crucial for its absorption and bioavailability, as well as its effectiveness in various formulations.
- Molecular Weight: The molecular weight of quercetin is approximately 302.24 g/mol, a factor that influences its distribution and metabolism within the body.
Formulations and Bioavailability
Poor Bioavailability of Standard Quercetin
Quercetin's bioavailability is complex, influenced largely by its poor water solubility, which results in low absorption and extensive metabolism, hence reducing its availability to exert biological effects. It is predominantly found in foods as glycosides, bound to sugar molecules, which impacts its absorption and subsequent bioavailability.
In a clinical study with a different formulation of quercetin (Quercetin Phytosome) a ~20 fold relative bioavailability compared to standard quercetin was measured. Given that Quercetin Phytosome contains ~40% quercetin, one can assume that the total bioavailability of standard quercetin is less than 2%. [7]
Quercetin, when consumed, undergoes an absorption process primarily in the small intestine. The glycosidic form of quercetin needs to be hydrolyzed by β-glucosidase to its aglycone form before absorption. Once hydrolyzed, it is absorbed through enterocytes via passive diffusion or through active transport mechanisms. The overall absorption of quercetin is estimated to be relatively low, varying between individuals and dependent on dietary matrix and presence of other flavonoids.
Once absorbed, quercetin undergoes extensive first-pass metabolism in the liver and intestines, where it is converted into various metabolites through glucuronidation, sulfation, and methylation. The extensive metabolism significantly reduces the concentrations of free quercetin in the plasma, limiting its bioavailability. The metabolites, however, may retain some biological activity and contribute to the overall effects of quercetin in the body.
It is suggested that individuals with poor vitamin C status might absorb quercetin better than those with adequate vitamin C levels. This could indicate a compensatory mechanism where the body might try to absorb more of certain beneficial compounds, like quercetin, in the absence of others, like vitamin C. However, the exact mechanisms or reasons behind this would need to be further explored. [8]
Formulation of Enhanced Bioavailability
The low bioavailability of quercetin raises questions about the clinical relevance of its potential health benefits observed in vitro and in animal studies. Therefore, several strategies have been explored to enhance the absorption and stability of quercetin. The following formulations are found on the market:
| Formulation | Alternative Names | Relative Bioavailability | Notes |
|---|---|---|---|
| Standard Quercetin | 1 (baseline) | Naturally occurring in various plants; serves as the baseline form of quercetin with poor solubility and bioavailability. | |
| Quercetin Dihydrate | – | Synthetic form with two water molecules associated; usually preferred for dietary supplements due to improved solubility. | |
| Quercetin Phytosomes | Quercetin-Phospholipid | 10-20 | Significantly increases oral bioavailability compared to standard quercetin. |
| Nanoparticle Formulations | Nanoquercetin, Quercetin Nanocapsules | Several-fold increase | Includes liposomal encapsulations; increased solubility and cellular uptake. |
| Quercetin LipoMicel | 8-9 | Encapsulating quercetin within a liquid micelle matrix | |
| Co-administration with Piperine | Bioavailability Enhanced Quercetin, Piperine-Quercetin Combination | Approximately 20% | Inhibits metabolism of quercetin, improving its bioavailability. |
Quercetin Phytosome

Quercetin Phytosome is a formulation where quercetin is bound to phospholipids, typically derived from sunflower or soy lecithin, to enhance its bioavailability and absorption. The relevant clinical study used Quercetin Phytosome (QUERCEFIT™) consisting of quercetin and sunflower lecithin in a 1:1 weight ratio along with about a fifth part of food-grade excipients that are added to improve the physical state of the product and to standardize it to an HPLC-measured total quercetin content of about 40%. In the clinical study, a ~20-fold increase in bioavailability was measured compared to standard quercetin with equal doses. [7]
| Quercetin 500 mg | Quercetin Phytosome 500 mg | Quercetin Phytosome 250 mg | |
|---|---|---|---|
| AUClast (min × ng/ml) | 4774.93 ± 1190.61 | 96,163.87 ± 9291.31 | 50,401.53 ± 6418.22 |
| Cmax (ng/ml) | 10.93 ± 2.22 | 223.10 ± 16.32 | 126.35 ± 14.79* |
| Tmax (min) | 290.00 ± 31.19 | 202.50 ± 35.97 | 228.75 ± 36.61 |
| t1/2 (min) | 375.63 ± 75.51 | 226.84 ± 8.13 | 201.63 ± 13.18 |
| MRT last (min) | 410.41 ± 24.24 | 372.94 ± 20.12 | 386.29 ± 21.04 |
Quercetin Phytosome overcomes the low bioavailability hurdle of quercetin and should help to fulfill the great health benefit potential of this flavonoid in the diet and as food supplements.
Quercetin LipoMicel

Quercetin LipoMicel is a novel formulation from Natural Factors designed to enhance the bioavailability of quercetin. LipoMicel encapsulates quercetin molecules within micelles, tiny spherical structures formed from natural, non-ionic surfactants. These micelles facilitate the solubilization of quercetin, allowing it to be more readily absorbed in the gastrointestinal tract.
In a study sponsored by Natural Factors, the bioavailability of Standard Quercetin, Quercetin Phytosome and Quercetin LipoMicel was evaluated. The results indicated that Quercetin LipoMicel had a relative bioavailability to Standard Quercetin of 8.5, while Quercetin Phytosome exhibited a slightly higher value of 8.9. Notably, LipoMicel maintained more consistent levels of quercetin in the system over a 24-hour period. [9]
In a second study, the blood concentrations of quercetin in healthy participants after the administration of standard quercetin and LipeMice quercetin was evaluated with over 72 hours and 4 doses in total. In contrast to other studies, the administration was given after 10 hours of fasting and continued fasting for 4 hours afterward to reduce potential interactions with food components like fiber and fat. Within the first 24 hours, LipeMice (500mg) had a significantly 7-fold increased blood concentrations of quercetin compared to standard quercetin (500 mg). LipeMice administered at a double dose (1000 mg) achieved 15-fold higher absorption, LipeMice tested at half a dose of standard quercetin increased concentration by approx. 3-fold. Quercetin blood concentrations were attained over 72 hours. [10]
| Study 1 [9] | Study 2 (with fasting) [10] | ||||||
|---|---|---|---|---|---|---|---|
| Standard
500mg |
LipoMicel
500mg |
Phytosome
500mg |
Standard
500mg |
LipoMicel
250mg |
LipoMicel
500mg |
LipoMicel
1000mg | |
| AUC0-24 (ng h/ml) | 172.87 ± 12.21 | 1477.27 ± 25.57 | 1536.00 ± 115.66 | 77.3 ± 6.1 | 197.0 ± 8.0 | 543.1 ± 20.3 | 1128.8 ± 52.3 |
| Cmax (ng/ml) | 19.77 ± 27.29 | 182.85 ± 106.64 | 150.27 ± 61.43 | 6.8 ± 9.2 | 15.4 ± 22.0 | 52.5 ± 51.7 | 150.4 ± 117.6 |
| Tmax (hours) | 2 ± 0.15 | 0.5 ± 0.02 | 1 ± 0.30 | 3.0 ± 3.2 | 1.0 ± 2.7 | 5.9 ± 2.7 | 0.9 ± 0.2 |
| t1/2 (hours) | 11.66 ± 0.55 | 8.29 ± 0.49 | 6.02 ± 0.52 | ||||
Clinical Applications and Effects on Longevity
Quercetin has been the focus of numerous clinical studies aiming to understand its potential applications and efficacy in addressing age-related conditions and promoting longevity due to its diverse biological activities.
- Anti-inflammatory Effects
- Quercetin’s potent anti-inflammatory properties have led to its investigation for use in chronic inflammatory conditions such as arthritis and cardiovascular diseases. It modulates inflammatory pathways by inhibiting the release of pro-inflammatory cytokines and reducing the activation of inflammatory cells.
- Antioxidant Properties
- The antioxidant activity of quercetin is attributed to its ability to neutralize free radicals and reduce oxidative stress, potentially mitigating aging-related cellular damage and dysfunction. Its antioxidant properties are linked to a reduction in the risk of chronic conditions such as cancer and neurodegenerative diseases.
- Impact on Cellular Senescence
- Research has indicated that quercetin may delay cellular senescence by modulating senescence-associated signaling pathways and reducing the accumulation of senescent cells, potentially impacting aging processes and age-related diseases.
- Clinical Studies and Trials related to Aging
- Several clinical trials and studies have been conducted to assess the safety and efficacy of quercetin supplementation in age-related conditions. The data, however, are still inconclusive, necessitating further well-designed studies to establish its therapeutic benefits in aging and longevity comprehensively.
- Role as an NAD+ Booster
- Quercetin has been identified as a potential NAD+ booster. Studies have demonstrated that quercetin can inhibit the activity of NADase (CD38), an enzyme responsible for the degradation of NAD+, thus potentially increasing the levels of NAD+ in cells. Elevated NAD+ levels are associated with improved mitochondrial function, enhanced cellular metabolism, and reduced oxidative stress, all of which are key components in the aging process.
Dosage and Administration
Exploring the optimal dosage and various administration forms is crucial for leveraging the potential benefits of Quercetin. Given the variances in individual responses and bioavailability, establishing the right dosage is paramount.
Recommended Dosages
Typical supplemental dosages of quercetin range from 500 to 1000 mg per day, usually divided into multiple doses. However, optimal dosages may vary based on individual health conditions, goals, and sensitivities, and consultation with a healthcare provider is advised for personalized recommendations.
Administration Forms and Bioavailability Enhancement
Quercetin is available in various forms, including tablets, capsules, and powders. To enhance its bioavailability, it is often formulated with bioflavonoids or bromelain, or encapsulated in liposomes or phytosomes. Different formulations may affect the absorption and efficacy of quercetin, and choosing the right form is crucial for optimal results.
Safety and Side Effects
Understanding the safety profile and potential side effects of Quercetin is vital for informed supplementation. While generally regarded as safe, quercetin may cause adverse reactions in certain situations or populations.
In 2010, the FDA acknowledged high-purity quercetin (≥ 99.5% quercetin) as Generally Recognized as Safe (GRAS) for use as an ingredient in various specified food categories at levels up to 500 milligrams per serving.[6]
Known Side Effects
In some individuals, quercetin supplementation can lead to side effects such as headaches, stomach pain, and tingling of the extremities. Rarely, it may cause kidney damage at high doses.
Interactions with Medications and Other Supplements
Quercetin has the potential to interact with various medications, including antibiotics and blood pressure medications, potentially altering their effects. Additionally, its interaction with other supplements, particularly those with similar biological effects, needs careful consideration to avoid cumulative effects or imbalances.
Safety Precautions and Contraindications
Individuals with kidney conditions, pregnant or breastfeeding women, and those on specific medications should consult healthcare providers before starting quercetin supplementation. Proper dosage and adherence to safety precautions are crucial to minimize the risk of adverse reactions.
Conclusions and Future Directions
Quercetin has garnered substantial attention in the realm of longevity and health due to its multifaceted biological activities, including antioxidant, anti-inflammatory, and potential anti-aging properties.
Summary of Key Findings
Quercetin’s diverse pharmacological properties, such as modulation of inflammatory pathways, neutralization of free radicals, and potential impact on cellular senescence, offer promising avenues for addressing age-related conditions and promoting health and longevity. However, the conclusive benefits and optimal dosages in humans are yet to be firmly established.
Gaps in Current Knowledge and Research
While quercetin’s therapeutic potential is evident from preclinical and some clinical studies, significant gaps persist in our understanding of its precise mechanisms of action, long-term safety, and efficacy in humans. Further well-designed clinical trials and comprehensive studies are imperative to bridge these gaps and validate quercetin's roles in human health and longevity.
Future Directions and Potential Implications
The ongoing and future research on quercetin is poised to explore novel formulations and delivery methods to improve its bioavailability and therapeutic efficacy. The elucidation of its molecular targets and mechanisms will facilitate the development of targeted interventions for age-related diseases and conditions, potentially impacting healthcare approaches and strategies for healthy aging.
Quercetin remains a compelling subject in the longevity and health science field, with its future promising to unveil deeper insights into its biological activities and therapeutic potentials, possibly leading to innovative solutions for aging and age-associated ailments.
See Also
- Wikipedia - Quercetin
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 USDA Database for the Flavonoid Content of Selected Foods, Release 3, http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/Flav/Flav_R03.pdf
- ↑ Slimestad R et al.: Onions: a source of unique dietary flavonoids. J Agric Food Chem 2007. (PMID 17997520) [PubMed] [DOI] Onion bulbs (Allium cepa L.) are among the richest sources of dietary flavonoids and contribute to a large extent to the overall intake of flavonoids. This review includes a compilation of the existing qualitative and quantitative information about flavonoids reported to occur in onion bulbs, including NMR spectroscopic evidence used for structural characterization. In addition, a summary is given to index onion cultivars according to their content of flavonoids measured as quercetin. Only compounds belonging to the flavonols, the anthocyanins, and the dihydroflavonols have been reported to occur in onion bulbs. Yellow onions contain 270-1187 mg of flavonols per kilogram of fresh weight (FW), whereas red onions contain 415-1917 mg of flavonols per kilogram of FW. Flavonols are the predominant pigments of onions. At least 25 different flavonols have been characterized, and quercetin derivatives are the most important ones in all onion cultivars. Their glycosyl moieties are almost exclusively glucose, which is mainly attached to the 4', 3, and/or 7-positions of the aglycones. Quercetin 4'-glucoside and quercetin 3,4'-diglucoside are in most cases reported as the main flavonols in recent literature. Analogous derivatives of kaempferol and isorhamnetin have been identified as minor pigments. Recent reports indicate that the outer dry layers of onion bulbs contain oligomeric structures of quercetin in addition to condensation products of quercetin and protocatechuic acid. The anthocyanins of red onions are mainly cyanidin glucosides acylated with malonic acid or nonacylated. Some of these pigments facilitate unique structural features like 4'-glycosylation and unusual substitution patterns of sugar moieties. Altogether at least 25 different anthocyanins have been reported from red onions, including two novel 5-carboxypyranocyanidin-derivatives. The quantitative content of anthocyanins in some red onion cultivars has been reported to be approximately 10% of the total flavonoid content or 39-240 mg kg (-1) FW. The dihydroflavonol taxifolin and its 3-, 7-, and 4'-glucosides have been identified in onions. Although the structural diversity of dihydroflavonols characterized from onions is restricted compared with the wide structural assortment of flavonols and anthocyanins identified, they may occur at high concentrations in some cultivars. From bulbs of the cultivar "Tropea", 5.9 mg of taxifolin 7-glucoside and 98.1 mg of taxifolin have been isolated per kilogram of FW.
- ↑ Mitchell AE et al.: Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J Agric Food Chem 2007. (PMID 17590007) [PubMed] [DOI] Understanding how environment, crop management, and other factors, particularly soil fertility, influence the composition and quality of food crops is necessary for the production of high-quality nutritious foods. The flavonoid aglycones quercetin and kaempferol were measured in dried tomato samples (Lycopersicon esculentum L. cv. Halley 3155) that had been archived over the period from 1994 to 2004 from the Long-Term Research on Agricultural Systems project (LTRAS) at the University of California-Davis, which began in 1993. Conventional and organic processing tomato production systems are part of the set of systems compared at LTRAS. Comparisons of analyses of archived samples from conventional and organic production systems demonstrated statistically higher levels (P < 0.05) of quercetin and kaempferol aglycones in organic tomatoes. Ten-year mean levels of quercetin and kaempferol in organic tomatoes [115.5 and 63.3 mg g(-1) of dry matter (DM)] were 79 and 97% higher than those in conventional tomatoes (64.6 and 32.06 mg g(-1) of DM), respectively. The levels of flavonoids increased over time in samples from organic treatments, whereas the levels of flavonoids did not vary significantly in conventional treatments. This increase corresponds not only with increasing amounts of soil organic matter accumulating in organic plots but also with reduced manure application rates once soils in the organic systems had reached equilibrium levels of organic matter. Well-quantified changes in tomato nutrients over years in organic farming systems have not been reported previously.
- ↑ Petrus K et al.: Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry. Anal Bioanal Chem 2011. (PMID 21229237) [PubMed] [DOI] The analysis of flavonoids in unifloral honeys by high-performance liquid chromatography (HPLC) coupled with coulometric electrode array detection (CEAD) is described. The compounds were extracted by a nonionic polymeric resin (Amberlite XAD-2) and then separated on a reversed phase column using gradient elution. Quercetin, naringenin, hesperetin, luteolin, kaempferol, isorhamnetin, and galangin were detected in a coulometric electrode array detection system between +300 and +800 mV against palladium reference electrodes, and their presence was additionally confirmed by HPLC coupled with electrospray ionization mass spectrometry. The method was applied to analysis of 19 honeys of different varieties and origin. The limits of detection and quantitation ranged between 1.6 and 8.3 μg/kg and 3.9 and 27.4 μg/kg, respectively. The recoveries were above 96% in fluid and above 89% in creamy honeys. Some of these honeys (melon, pumpkin, cherry blossom, dandelion, maple, and pine tree honey) were investigated for their flavonoid content and profile for the first time. Differences between honeys were observed both in flavonoid concentrations and in the flavonoid profiles. The flavonoid concentrations ranged from 0.015 to 3.4 mg/kg honey. Galangin, kaempferol, quercetin, isorhamnetin, and luteolin were detected in all investigated honeys, whereas hesperetin occurred only in lemon and orange honeys and naringenin in lemon, orange, rhododendron, rosemary, and cherry blossom honeys.
- ↑ Flavonoids, http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids
- ↑ 6.0 6.1 GRN No. 341 (Quercetin), https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=341
- ↑ 7.0 7.1 7.2 7.3 Riva A et al.: Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur J Drug Metab Pharmacokinet 2019. (PMID 30328058) [PubMed] [DOI] [Full text] BACKGROUND AND OBJECTIVES: The importance of quercetin and flavonoids in the diet and as food supplements is well known, and literature studies support their potential use to treat several human diseases. Many beneficial properties have been described for quercetin, so much effort has been directed into overcoming the major drawbacks of this natural compound-its poor solubility and low oral absorption. The aims of this study were to compare a new food-grade lecithin-based formulation of quercetin, Quercetin Phytosome®, to unformulated quercetin in terms of solubility in simulated gastrointestinal fluids and oral absorption in a randomized crossover pharmacokinetic study of healthy volunteers. METHODS: The solubility of the new formulation was determined by in vitro incubation in simulated gastrointestinal fluids, and quercetin was detected by ultra performance liquid chromatography. A single-dose, randomized, six-sequence/three-period crossover clinical trial (3 × 3 × 3 crossover design) with a balanced carryover effect was conducted in healthy volunteers under fasting conditions. Twelve healthy volunteers of both sexes with an age range of 18-50 years were recruited; one dose of quercetin and two different doses of Quercetin Phytosome were administered orally as film-coated tablets. Pharmacokinetic samples were collected at twelve time points (from 0 h to 24 h) after administration, and quercetin levels were measured by HPLC/MS/MS. Data were analyzed using the Phoenix WinNonlin (v.6.4) software package, and the most significant pharmacokinetic parameters were calculated. Statistical analysis involved performing a two-way ANOVA with repeated measures followed by post hoc analysis (Tukey's test). RESULTS: Significant improvements in both in vitro solubility and oral absorption (in terms of both exposure and maximum concentration achieved) by healthy volunteers in a human clinical study were obtained with the Quercetin Phytosome formulation as compared to unformulated quercetin. CONCLUSIONS: A more soluble formulation of quercetin based on lecithin, Quercetin Phytosome, has recently been developed, and was found to facilitate the attainment of very high plasma levels of quercetin-up to 20 times more than usually obtained following a dose of quercetin-when the novel formulation was administered orally in human volunteers, and it did not have any notable side effects. These results suggest that Quercetin Phytosome allows the oral administration of quercetin in a safe and bioavailable manner, thus facilitating the effective utilization of this natural compound to treat various human diseases.
- ↑ Guo Y et al.: Quercetin bioavailability is associated with inadequate plasma vitamin C status and greater plasma endotoxin in adults. Nutrition 2014. (PMID 25280405) [PubMed] [DOI] OBJECTIVE: Quercetin bioavailability exhibits high interindividual variation for reasons that remain unclear. We conducted a 24-h pharmacokinetic study to investigate whether individual differences in circulating antioxidants, oxidative stress and inflammation, and intestinal permeability affect quercetin bioavailability. METHODS: Healthy adults (n = 9 M/7 F; 34.3 ± 4.5 y; 27.0 ± 1.7 kg/m(2)) ingested 1095 mg quercetin aglycone with a standardized meal. Plasma antioxidants, biomarkers of oxidative stress and inflammation, and endotoxin were measured at baseline (0 h), and quercetin and its methylated metabolites isorhamnetin and tamarixetin were measured at timed intervals for 24 h. RESULTS: Plasma pharmacokinetics of quercetin, isorhamnetin and tamarixetin were highly variable between participants (CVinter = 37-96%). Plasma vitamin C concentrations (34.6 ± 2.5 μmol/L), but no other antioxidants, were inversely correlated to the Cmax and AUC0 to 24 h of total quercetin (Qtotal; sum of quercetin, isorhamnetin and tamarixetin; r = -0.52 to -0.53; P < 0.05). Plasma endotoxin (0.13 ± 0.01 EU/mL), a surrogate marker of intestinal permeability, was correlated to Qtotal Cmax (r = 0.45; P < 0.05) and tended to be correlated to Qtotal AUC0 to 24 h (r = 0.38; P = 0.07). Additionally, vitamin C was inversely related to C-reactive protein, myeloperoxidase, and endotoxin (r = -0.46 to -0.55; P < 0.05), whereas endotoxin was positively correlated to C-reactive protein (r = 0.73; P < 0.05). CONCLUSIONS: These findings suggest that vitamin C status and plasma endotoxin may be associated with interindividual variations in quercetin bioavailability. Greater quercetin absorption and bioavailability may be associated with poor vitamin C status and increased intestinal permeability in healthy adults.
- ↑ 9.0 9.1 9.2 https://jnhpresearch.com/index.php/jnhpr/article/view/17/24
- ↑ 10.0 10.1 Solnier J et al.: A Pharmacokinetic Study of Different Quercetin Formulations in Healthy Participants: A Diet-Controlled, Crossover, Single- and Multiple-Dose Pilot Study. Evid Based Complement Alternat Med 2023. (PMID 37600550) [PubMed] [DOI] [Full text] This study aimed to evaluate the blood concentrations of quercetin in healthy participants after the administration of different formulations in single- and multiple-dose phases. Ten healthy adults (males, 5; females, 5; age 37 ± 11 years) participated in a diet-controlled, crossover pilot study. Participants received three different doses (250 mg, 500 mg, or 1000 mg) of quercetin aglycone orally. In the single-dose study, blood concentrations (AUC0-24 and Cmax) of standard quercetin were compared with those of LipoMicel®-a food-grade delivery form of quercetin. In the multiple-dose study, blood concentrations of formulated quercetin were observed over 72 h, after repeated doses of LipoMicel (LM) treatments. The AUC0-24 ranged from 77.3 to 1128.9 ng·h/ml: LM significantly increased blood concentrations of quercetin by 7-fold (LM 500) compared to standard quercetin, when tested at the same dose, over 24 h (p < 0.001); LM administered at a higher dose (LM 1000) achieved 15-fold higher absorption (p < 0.001); LM tested at half a dose of standard quercetin increased concentration by approx. 3-fold (LM 250). Quercetin blood concentrations were attained over 72 h. The major metabolites measured in the blood were methylated, sulfate, and glutathione (GSH) conjugates of quercetin. Significant differences in concentrations between quercetin conjugates (sulfate vs. methyl vs. GSH) were observed (p < 0.001). Data obtained from this study suggest that supplementation with LipoMicel® is a promising strategy to increase the absorption of quercetin and its health-promoting effects in humans. However, due to the low sample size in this pilot study, further research is still warranted to confirm the observations in larger populations. This trial is registered with NCT05611827.